Electric Charge and Coulomb’s Law
Let’s dig a little deeper into charge, which is the foundation of electricity. This section covers basic knowledge about what an electric charge is and details how to calculate the amount of charge using Coulomb’s Law.
What is electric charge?
Electric charge is the amount of electricity a charged object has. If an object has more electrons than protons, it is negatively charged. If an object has fewer electrons than protons, it is positively charged. Electric charge is represented by the symbol Q and is measured in a unit called coulombs (C). If the two charges Q1 and Q2 are separated by a distance r [m], then the force working between the charges, represented as F [N], is given by Coulomb’s Law as follows:
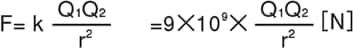
In general media, the following formula applies: (εs = relative permittivity of medium)
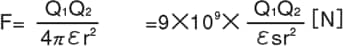
Coulomb’s Law
When two charged objects come close to each other, a force of repulsion works between charges of the same polarity while a force of attraction works between charges of different polarity.
This electric force is called Coulomb force (unit is [N]). The relation between the amount of electric charge and this force is indicated by Coulomb’s Law.
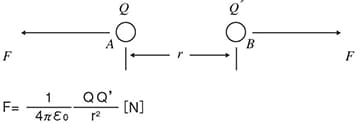
An electric charge in an object so tiny that its size is not measurable is called a point charge. If point charge A with an amount of charge Q [C] is approached by point charge B with an amount of charge Q’ [C] in a vacuum at a distance r [m], the magnitude of static force can be calculated using Coulomb’s Law as being inversely proportional to the square of the distance between A and B (r [m]) and being directly proportional to the product of A’s amount of charge and B’s amount of charge.
The proportionality constant ε0 is referred to as vacuum permittivity (8.85×10-12 [F/m]: farad per metre).
The Coulomb force is a force of repulsion when the two charges are of the same polarity and a force of attraction when the polarity is different. Dividing this by acceleration of gravity 9.8 [m/s2] produces [kg].
Example:
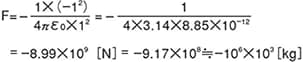
The magnitude of static force (F) working between two point charges 1 [C] and -1 [C] separated by a distance of 1 [m] is represented by the following formula.
According to this formula, the acting force is approximately 1 million tons, which is equivalent to a force that could lift a weight of 1 million tons. A coulomb [C] is too large a unit to use in realistic situations, so the amount of charge when a 1 [m] square polymer film is frictionally charged, which is around 10-5 [C], is used.
Coulomb’s Law
Coulomb’s Law is said to have been discovered by ancient Greek philosopher Thales (BC. 640 to 546). However, it was announced in 1785 that Coulomb (1739 to 1806) had generalised this theorem into a law.